Journal of Creation 32(1):48–53, April 2018
Browse our latest digital issue Subscribe
A naturalist’s nightmare
A review of A Fortunate Universe: Life in a finely tuned cosmos by Geraint F. Lewis and Luke A. Barnes
Cambridge University Press, UK, 2016

“We are such insignificant creatures on a minor planet of a very average star in the outer suburb of one of a hundred billion galaxies. So it is difficult to believe in a God that would care about us or even notice our existence” (Professor Stephen Hawking, FRS, cosmologist).1
“… humanity appears to be part of a remarkable set of circumstances involving a special time around a special planet, which orbits a special star, all within a specially constructed Universe” (Professor Brian Schmidt, astrophysicist and Nobel Prizewinner (foreword, p. xii)).
This is a remarkable book, full of helpful information for those seeking to understand the extent to which our universe is finely tuned for life. The authors are well qualified to write on the subject. Geraint Lewis is Professor of Astrophysics at the Sydney Institute for Astronomy and Luke Barnes has a Ph.D. in astronomy from Cambridge University in the UK. Some of the material is hard going for the non-specialist and can be very detailed and longwinded. However, there is still much that can be grasped by those who have a basic understanding of chemistry and physics.
Unfortunately, and despite its many problems,2 the authors unquestioningly accept big bang theory as the explanation for the universe. Consequently, the reader must make a conscious effort to distinguish between the fine-tuning needed for life generally, and that required to support the big bang. To avoid confusion in this review, care has been taken to separate the two issues, the general case being addressed first and big bang fine-tuning second. In both cases, however, the fine-tuning arguments are those of Lewis and Barnes, not the reviewer.
Masses of fundamental particles

Atoms are made up of a nucleus with electrons in orbit around it. The nucleus contains protons and neutrons which, in turn, are made up of quarks—‘up quarks’ and ‘down quarks’. A proton comprises two up quarks and one down quark, and a neutron two down quarks and one up quark.
The masses of the quarks must be just right to sustain life (pp. 48–53). Neutrons are slightly heavier than protons, so a lone neutron will decay into a proton + electron + antineutrino in a mean lifetime of about 15 minutes. In the nucleus, they are stabilized by the strong nuclear force. But if the mass of the down quark were increased by a factor of three, neutrons would decay even in a nucleus, leaving a universe containing only hydrogen, i.e. one dominated by protons.
Decrease the mass of the down quark by merely 8%, and protons in atoms will capture the electrons in orbit around them, leaving a universe dominated by neutrons. Increasing the mass of the electron would cause protons and electrons to spontaneously form neutrons, also leading to a neutron universe. In both proton-dominated and neutron-dominated universes we can say goodbye to the periodic table and, with it, the possibility of the molecules needed to support any lifeform.
The fine-tuning requirements associated with the masses of these fundamental particles are illustrated graphically in figure 1 where the small grey region shows the conditions required for chemistry to operate and the molecules needed for life. Its smallness, however, is not illustrated very clearly. The axes could legitimately be extended so that the white (representing sterile universes) covers many square kilometres, showing the grey area to be miniscule in comparison (pp. 52, 53).
Magnitudes of fundamental forces
In our universe there are four fundamental forces:
- gravity force
- electromagnetic force
- strong nuclear force
- weak nuclear force
While gravity and electromagnetism are easily observed, the strong and weak nuclear forces are more esoteric, operating within the very centres of atoms. The force due to gravity results in the attraction of matter to itself. Electromagnetic forces cause electrically charged particles to attract or repel one another, with like charges repelling and unlike charges attracting. It is this force that holds negatively charged electrons in orbit around their positively charged atomic nuclei. The strong nuclear force binds quarks together to form protons and neutrons. It also holds the atomic nucleus together, overcoming the tendency of the positively charged protons to push one another apart. The weak nuclear force is involved in radioactive beta decay and nuclear fusion.

The strong nuclear force needs to be strong in order to maintain the general stability of matter. For example, as the strong force is reduced, more and more elements become radioactive (due to alpha decay); and as protons and neutrons become free to escape from their nuclei, one kind of element will turn into another! However, if the strong nuclear force were just a little stronger, it would bind the diproton (i.e. cause two protons to stick together) leading to stellar instability—and our sun would burn through the entirety of its fuel in about a second. If it were just a little weaker, deuterium would unbind (i.e. fall apart), preventing the first nuclear reaction step in the sun3 (pp. 65–94). According to Professor Paul Davies, without deuterium, “It is doubtful if stable, long-lived stars could exist at all.”4
At the same time, the electromagnetic force needs to be relatively weak, so that the electrons orbiting atomic nuclei can be rearranged, facilitating chemical reactions and the forming of molecules. In fact, the energy needed to break a strong nuclear bond is about 20,000 times that needed to break an electromagnetic bond (pp. 73–74). Additionally, if the electromagnetic force were just a bit stronger, protons would become unstable leading to a universe full of neutrons and electrons and no elements.5 Referring to the fine-tuning required of the nuclear strong force and the electromagnetic force, Lewis and Barnes conclude: “Playing these forces off against one-another has a drastic effect on the universe, with an almost imperceptible region of stability” (p. 75). This is illustrated in figure 2.

Let’s now turn our attention to the force due to gravity (pp. 107–111). In order for stars (including our sun) to be stable, there must be a balance between the inward pull of gravity and the outward push arising from thermal pressure. If a ball of gas had too little mass, gravity would not squeeze it tightly enough to produce nuclear reactions and it would never shine as a star. If it had too much mass, the force of gravity would produce excessive nuclear reactions, causing the star to burn fuel too quickly, resulting in it blowing off its outer gas layers.
If the force due to gravity were weaker, stars would need to be larger in order to be stable. Such stars would be unsuitable as suns for habitable planets because they would produce low-energy photons such as infrared. These would simply warm the earth like an oven, making processes like photosynthesis unworkable. If the force due to gravity were stronger, stars would need to be smaller. These would, again, be unsuitable for life because they would emit high-energy photons such as gamma rays which would sterilise the earth.
Further to this, it is necessary to consider the electromagnetic force as this also determines the conditions in which nuclear reactions occur and whether a star will be stable. Figure 3 illustrates the extent of the fine-tuning required of the gravity force and the electromagnetic force.
Quantum mechanics and Planck’s constant
Most people have been taught that the atom is rather like a solar system: just as planets orbit our sun, so electrons orbit the atomic nucleus. (Although in the case of electrons, the centripetal force maintaining the orbit is electromagnetic rather than gravitational.) It’s not that simple however. Orbiting electrons would be expected to lose their energy by emitting radiation—and crash into the nucleus. The answer to this problem lies in the rather esoteric field of quantum mechanics.8 According to this, the electron is a wave which is constrained to fit within a given orbit. Its wave-like properties, however, are strange: the peaks and troughs indicate where the electron probably is and where it is probably going. It’s a rather uncertain world!
If this quantum weirdness were experienced in respect of larger objects, we would never know quite where things are or where they were going. Balls on a billiard table might behave strangely, “spreading out as they roll until it looked as if not one ball was rolling across the table, but a great number of balls, all partially penetrating into each other” (p. 190). The path of a football sailing through the air might be affected by anything else in the universe (p. 191).
The scale at which quantum mechanics becomes significant is determined by Planck’s constant which, in our universe, has the very small value of ≈ 6.6 x 10–34 J s. If it were zero, quantum mechanics would not operate and atoms would become unstable. If it were much larger, quantum weirdness would start to affect our everyday lives making the world in which we live unpredictable. Even the ability of DNA to store information would be compromised. Happily, Planck’s constant is just where we need it to be (p. 191).
Another consequence of quantum mechanics is that the atoms comprising matter are never still, even in the absence of a heat source. (For this reason, it would be impossible for something to be cooled to absolute zero.) In our universe, where electrons are much lighter than protons, this ‘quantum jiggling’ is quite gentle, enabling objects to exist in solid form. However, if the electron mass were within a factor of a hundred of the proton mass, everything would melt. Consequently, there would be no solid planets, no stable DNA molecules, and no life (pp. 56–58).
Fine-tuning and the big bang
It is here that Lewis and Barnes’ book becomes most useful as, in page after page after page, they explain how parameter after parameter after parameter would have had to be just right for the big bang to have produced a universe capable of sustaining life. In this review it is possible only to mention a few.
Light elements
According to big bang theory, only ‘light elements’ were formed in the first few minutes of the early universe, these being hydrogen and helium (along with small quantities of deuterium, lithium, and beryllium). In this brief period, the temperature is said to have been just right for nuclei to form from protons and neutrons with the amount of each element being determined by the four fundamental forces (pp. 77–79).
If the strong force were greater, nuclear reactions would have been much more efficient and all the hydrogen would have been burnt up, leaving a helium universe. No water (H20) could then form and there could be no carbon-based molecules involving hydrogen (which accounts for almost all carbon compounds). In fact, increasing the strong force by a factor of about 2 would result in the early universe burning 90% of its hydrogen. Reducing the weak force would also lead to a preponderance of helium due to the nascent universe containing roughly equal numbers of protons and neutrons. (Hydrogen atoms have just one proton and no neutron; helium atoms have equal numbers of protons and neutrons, i.e. two of each.) The gravitational force here is also significant as it constrains how fast the universe expands and therefore how fast it cools. Increasing the gravitational force has the same effect as reducing the weak force—the production of equal numbers of protons and neutrons leading to a helium universe.
The observed abundance of light elements in our universe is said to be prediction of big bang theory and therefore evidence for it. However, here Lewis and Barnes make a remarkable admission. Having first emphasised how “All four fundamental forces have a say in how much of each element is produced” (p. 76) they state:
“… changing the strengths of the fundamental forces can have a dramatic effect on the nuclear reactions in the very early Universe … if there were no nuclear reactions at all … then we’d be left with pure hydrogen … . We would, interestingly, be missing a crucial piece of evidence for Big Bang theory” (p. 77).
Hence, it would seem that this alleged evidence for the big bang is itself dependent upon fine-tuning!
The smoothness of the early universe
For stars (and ultimately) planets to have formed, the distribution of matter in the early universe would have needed to be just right. If too even, gravity would never have been sufficiently concentrated so as to draw gases together to form stars. In other words, a degree of ‘lumpiness’ is needed to provide the ‘seeds’ of galaxies. If too lumpy, however, stars would be too closely packed together and planets would not have stable orbits. To produce a universe like ours, the density of parts of the universe must have deviated from the average density by approximately 1 part in 100,000, a property referred to as Q. Decreasing Q to 1 part in 1,000,000 would result in no stars, planets, or life. Increasing Q to 1 part in 10,000 would result in nearby stars disrupting planets in their orbits, again making life impossible (pp. 169–170).
The cosmological constant
The cosmological constant describes a repulsive ‘antigravity’ force arising from ‘dark energy’, a mysterious property of space, sometimes referred to as ‘vacuum energy’, that many cosmologists understand to be causing an accelerated expansion of the universe. (The reality of ‘dark energy’ of course has been contested by creationists who have argued that it is yet another ‘fudge factor’ required to make the failing big bang theory fit with observations.9)
According to Lewis and Barnes, the cosmological constant is “just about the best fine-tuning case around” (pp. 163–164). Nobel laureate Steven Weinberg would agree and argues that, unless fine-tuned to 120 decimal places, “the universe either would go through a complete cycle of expansion and contraction before life could arise, or would expand so rapidly that no galaxies or stars could form”.10
Carbon and oxygen
According to big bang theory, Earth’s carbon and oxygen were originally produced inside stars. However, if the strong nuclear force were increased by a little more than 0.4%, stars would produce lots of carbon but not oxygen. Conversely, if it were reduced by the same amount, they would produce lots of oxygen but not carbon (p. 119). Similarly, a small change in the masses of quarks would destroy a star’s ability to produce both carbon and oxygen (p. 120). Without carbon, carbon-based life could not exist; without oxygen, there can be no water and no life as we know it.
Some have suggested that silicon could replace carbon as the basis for life-permitting molecules. Reality, however, is not so simple. Whereas carbon can form 29,019 compounds with hydrogen, silicon can form only 55. Silicon’s equivalent of CO2 (SiO2) is a solid crystal (p. 269). Lewis and Barnes do not dismiss the possibility of silicon-based life but argue that this would further constrain the fine-tuning parameters (p. 270).
Objections to fine-tuning
In the penultimate chapter, the authors deal comprehensively with objections to the view that fine-tuning is a reality, and demonstrate that these carry little weight. Interestingly, they comment:
“The fine-tuning of the Universe for life is unique in our experience for the strength of the opinions expressed. … Even those who don’t think fine-tuning means anything simply must enthusiastically explain to everyone, in great detail, exactly why it doesn’t mean anything.”
In response to the claim that evolution would adapt life to whatever conditions it finds, they answer that alternative universes are not just uninhabitable because of high levels of temperature, pressure, saltiness, or acidity; rather:
“… the extremes of parameter space … are disintegrating atoms, the cessation of all chemical reactions, the crush of a black hole, and the eternal loneliness of life in a universe where particles collide every trillion years or so” (p. 244).
Some dismiss fine-tuning, claiming that all examples involve changing just one variable and keeping the others fixed. Hence, they say, life-permitting universes might be common if the dials controlling a number of parameters were changed simultaneously. In response, the authors argue that:
“… spinning multiple dials is usually as destructive as spinning one. … Sure there are many dials. But there are also many requirements for life. Adding more dials opens up more space [i.e. more possible universes], but most of this space is dead. We see no trace whatsoever of a vast oasis of life” (pp. 256, 261).
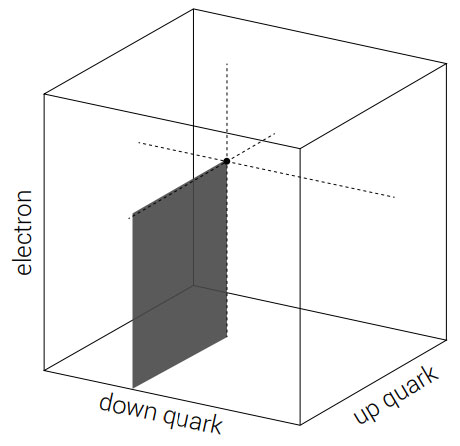
This they illustrate for the case of masses of the electron, down quark and up quark. (See figures 4 and 5, both of which show fine-tuning requirements independent of big bang theory.)

A designer?
Barnes leans towards the view that the fine-tuning is not accidental but purposeful. To him the universe “contains good things, like free moral agents and all that they can do and learn and appreciate”. These, he feels, reflect the intent of a creator (pp. 347–348). Barnes is quite well read on theistic arguments from a largely Thomistic perspective as well as atheistic responses. On his blog, he has been very critical of atheists such as Victor Stenger, Neil deGrasse Tyson, and Richard Carrier.
Lewis is more sceptical and argues that the presence of evil and suffering makes God’s existence unlikely: “I would expect a morally perfect being to create a morally perfect universe” (p. 346). He sees a multiverse as a more probable explanation for fine-tuning:
“Ours is but one of a vast sea of universes, and each with differing laws of physics and properties of matter, set at their birth through some cosmic roll of the dice … we find ourselves in one of the extremely few universes that can support life—the anthropic principle in action” (p. 353).
Lewis, however, does not appear to have done his homework. The Bible makes clear that the presence of evil and suffering is due to man’s sin which led to God cursing an original perfect creation. Realistically, multiverse thinking can have no place in science. Apart from being unobservable (and therefore untestable) it logically leads to the view that no data set should be regarded as evidence for anything. In a multiverse it could always occur by chance! In essence, the “anthropic principle in action” requires the universe to be finely tuned for life as, otherwise, we wouldn’t be here to observe it. However, this is really just a truism and fails to explain why it is so.
Conclusion
A life-sustaining universe requires a number of fundamental physical constants to be very precisely determined, and creationists rightly view this as evidence of intelligent design.
In addition, the big bang could not produce a life-sustaining universe unless many additional characteristics were exquisitely fine-tuned. This is so improbable that, to any reasonable mind, such a naturalistic explanation must be seen to be utterly, utterly implausible.
References and notes
- Cited by Gosh, P., Neural Suitcase Tells the Tales of Many Minds, Partridge Publishing, India, ch. 69, 2014. Return to text.
- Wieland, C., Secular scientists blast the big bang: what now for naïve apologetics? Creation 27(2):23–25, 2005; creation.com/bigbangblast. Return to text.
- Tegmark, M., Is ‘the theory of everything’ merely the ultimate ensemble theory? Annals of Physics 270:1–51, 1998; arxiv.org/pdf/gr-qc/9704009.pdf. Return to text.
- Davies, P.C.W., The Accidental Universe, Cambridge University Press, UK, p. 70, 1982. Return to text.
- Barnes, L.A., The Fine-Tuning of the Universe for Intelligent Life, Publications of the Astronomical Society of Australia 29:529–564, 2012; www.cambridge.org. Return to text.
- Tegmark, ref. 3, fig. 5, p. 16. Return to text.
- Barnes, ref. 5, fig. 6, p. 549. Return to text.
- Sarfati, J., Should creationists accept quantum mechanics? J. Creation 26(1):116–123, 2012; creation.com/qm. Return to text.
- Hartnett, J.G., Dark energy and the elusive chameleon—more darkness from the dark side, creation.com/dark-energy, 8 October 2015. Return to text.
- Weinberg, S., Facing Up: Science and its cultural adversaries, Harvard University Press, Cambridge, MA, pp. 80–81, 2001. Return to text.









Readers’ comments
Comments are automatically closed 14 days after publication.