Feedback archive → Feedback 2020
Amino acids produced in hydrothermal vent?
Does this help chemical evolution?

Materialists must believe that life arose from non-living chemicals by chemical evolution. This remains an intractable problem for materialists, because they can’t invoke natural selection as they do, however implausibly, for biological evolution. While natural selection is a proven fact discovered by creationists before Darwin, it means differential reproduction, so it can’t be used to explain how the first self-reproducing entity arose. So evolutionists can invoke only time, matter, and energy, not natural selection.
But from time to time, we receive questions about papers that purport to prove that chemical evolution is possible. One way to look at them is that they are concessions to the fact that previous papers had not solved the problem. Similarly, when an article claims to have found “the missing link”, we must wonder whether previous claims to find the missing link were valid.
The following is an example of a recent inquiry about an origin-of-life paper. Dr Jonathan Sarfati answers both the general principles and this specific case.
Hello, I read almost anything you post in Creation.com, but an atheist friend asked me about this article.1
This claims:
a natural chemical reaction that occurs below the sea floor makes the amino acid tryptophan without biological input. This finding reveals a process that might have helped life on Earth to begin.
Could you guys help me to this answer?
Thank you for writing to CMI about this 2018 article, which is a comment on this paper.2
First, my colleague Shaun Doyle has written a helpful article for anyone encountering articles like this: Reading ‘origin of life’ research: How to read the secular literature on chemical evolution (i.e. ‘abiogenesis’) critically. We would like all inquirers to consult this, because it would not only help with the paper in question, but future papers as well.
Second, my colleague Dr Don Batten has written a comprehensive overview of the problems of chemical evolution (‘abiogenesis’). Abiotic production of amino acids, as claimed in the paper you sent, is the first of the hurdles and maybe even the least difficult, which says a lot about the difficulty of the remaining steps.
Specifics
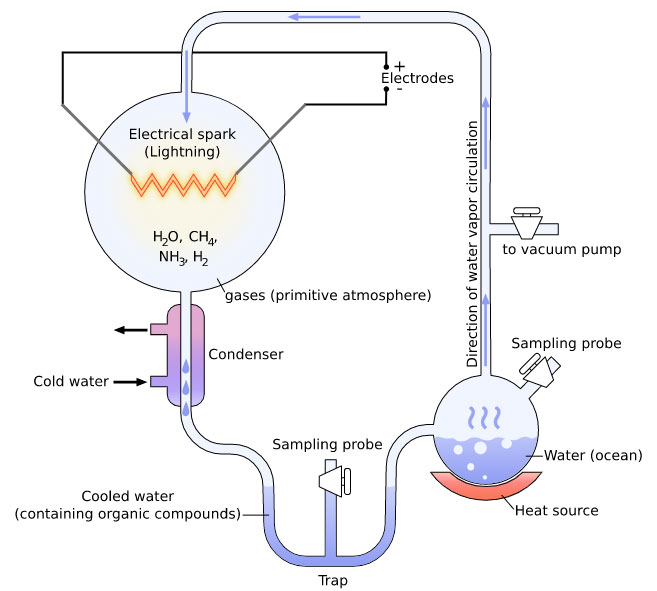
When it comes to the paper in question, even if tryptophan were formed under such conditions, how long would it last, given that biomolecules are unstable at high temperatures? We wrote about this a while back— Hydrothermal origin of life? As evidence for instability, we cited the famous chemical evolution pioneer Stanley Miller, of the eponymous Miller–Urey experiment.
It’s notable that the original paper tacitly agreed with this problem:
These spectral features are indicative of the presence of tryptophan to which the band at 358 nm can be assigned, and of indole, skatole and hydroxyanthranilic acid, the fluorescence spectra of which were reported previously. These three latter compounds may correspond to products of either natural or UV-induced degradation of tryptophan, although indole can also be an intermediate in the abiotic synthesis of tryptophan. (Italics added, references omitted.)
Note that they wouldn’t find what they agree are likely breakdown products of tryptophan unless this in fact was breaking down!
Another statement in the paper reveals another problem: life requires a huge number of different types of biomolecules, but no experiment produces more than a small fraction of these. The required conditions to produce some essential biomolecules are incompatible with those needed to produce others. For example, alkaline solutions, such as proposed in this scenario, would hydrolyze the amino acids serine, threonine, cystine, cysteine, and arginine3, and destroy aldose sugars—including ribose and glucose—via the Cannizzaro reaction, which converts two molecules of an aldehyde to an alcohol and an acid. These destructive processes would occur much more quickly at hydrothermal temperatures. In this case, it was the absence of certain biomolecules that was used as evidence that the tryptophan was produced abiotically:
In all the areas where saponite and fragment ions characteristic of tryptophan were detected, TOF-SIMS analysis did not provide any evidence for the presence of biomarkers, such as hopanoids, cholestane, pristane, squalane, lycopene, or ß-carotane, which are constituents of marine-dissolved organic carbon or of deep microbial communities.
Thus I think these scientists have made a strong case for abiotic production of tryptophan. But in making this case, they have shown why this is irrelevant for chemical evolution—so few of the required biomolecules are produced!
The paper also shows a major problem with this and many other papers purporting to find the ‘building blocks’ of life. That is, they are not in fact building anything! One reason is instability of building blocks in general, not just tryptophan as noted above. Another one is how they would join up to form proteins — see Origin of life: the polymerization problem.
In that article you sent me, another problem is hinted at. The author suggested some follow-up research:
To extend Ménez and co-workers’ report of the abiotic synthesis of tryptophan, future studies at Lost City should try to collect adequate volumes of fluid to determine a structural property, called chirality, of the tryptophan present. Molecules can exist in two mirror-image chiral forms. Synthesis of a molecule by a non-biological process generally results in equal proportions of these two forms [racemic mixture, racemate], whereas biologically synthesized amino acids are usually made in predominantly one form or the other [homochiral].
But this tacitly admits yet another problem: the origin of biological homochirality. It agrees that natural processes will produce a racemate—so much so that it would be diagnostic of abiotic origin. This would be good supporting evidence, but not conclusive. Homochiral biological forms can racemize, especially under high temp, and even more so if conditions are alkaline as per these conditions. This means that racemic tryptophan could have a biological origin.
This is a further difficulty for chemical evolution. Even if there were some way of obtaining homochiral amino acids from an original racemate, they would need to be used before they racemized.
Last, the conditions of hydrothermal vents are incompatible with conditions other chemical evolutionist claimed were necessary for origin of life. E.g. John Sutherland, whose claims about supposed prebiotic synthesis of nucleotides we have addressed elsewhere,4 argues that his own scenario would be impossible in a hydrothermal vent:
It is not definitive proof that the building blocks of biology arose in this way, but it is compelling and indicates that the requirements for these reactions to take place should be used to constrain geochemical scenarios on the early Earth. A requirement for ultraviolet irradiation to generate hydrated electrons would rule out deep sea environments. This, along with strong bioenergetic and structural arguments, suggests that the idea that life originated at vents should, like the vents themselves, remain “In the deep bosom of the ocean buried.” The chemistry places certain demands on the environment of the early Earth: for example, the high concentrations of certain species through evaporation of solutions.5
Conclusion
This is an example of a point we emphasize: the difference is often not with the facts adduced by evolutionists, but their interpretation due to their different axioms or starting assumptions. In this case, the scientists have made a good case for abiotic production of tryptophan: multiple lines of evidence, and considering alternatives. The difference is that they interpret these facts as supportive of chemical evolution; a better interpretation of the same facts is that they show how life could not have evolved under these conditions.
References and notes
- Baross, J.A., The rocky road to biomolecules, Nature, News and Views, 7 Nov 2018. Return to text.
- Ménez, B. and 8 others, Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere, Nature 564:59–63, Nov 2018 | doi:10.1038/s41586-018-0684-z. Return to text.
- Thaxton, C. et al., The Mystery of Life’s Origin: the continuing controversy, p. 105, Discovery Institute Press, 2020. This is an updated and expanded edition of the 1984 book The Mystery of Life’s Origin: Reassessing Current Theories—see review by Dr Ralph Matthews, J. Creation 9(1):55–26, 1995. Return to text.
- See also: Origin-of-Life researcher admits, It’s “A Long, Long Way to LUCA”, evolutionnews.org, 3 July 2017. Return to text.
- Sutherland, J.D., Studies on the origin of life—the end of the beginning, Nature Reviews Chemistry 1:12, 2017. Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.