Galápagos finches, rapid speciation, and recent creation

We have written about Darwin’s finches multiple times over the years, but new research has recently shined a brighter light on them. Instead of being the poster children of Darwinian evolution, they testify to recent creation! Darwin’s finches are part of the finch-sparrow supergroup of approximately 1,000 species. Being that only seven pairs of each ‘kind’ of bird were on the Ark (Genesis 7:3), these species clearly radiated quickly. Yet, they are also the same ‘kind’. In fact, it is abundantly clear that God created organisms to change and adapt over time. Thus, it should not surprise us to find many examples of speciation, hybridization, even of natural selection, among Darwin’s finches. Indeed, we have.
A little explanation might be helpful here. When biblical creationists talk about the ‘created kinds’, we are referring to the groups of organisms that God created in Genesis 1. For example:
So God created the great sea creatures and every living creature that moves, with which the waters swarm, according to their kinds, and every winged bird according to its kind. And God saw that it was good. (Genesis 1:21)
The Hebrew word for ‘kind’ is mîn (מִין). The word for ‘create’ is bara (ברא). Thus, we can also use the made-up word baramin when talking about created kinds. The Bible does not tell us how many kinds God created, how different they were to one another, or how much genetic diversity was included in each kind. These are things that we get to explore today and, based on evidence of rampant hybridization among the birds in this group, all finches and sparrows appear to belong to one baramin.
It is also important to note that the rise of new ‘species’ among the various created kinds is an important part of the biblical model. The appearance of new species is not evolution, nor is it proof of evolution. As Dr Carl Wieland said a quarter of a century ago:
Poorly informed anti-creationist scoffers occasionally think they will ‘floor’ creation apologists with examples of ‘new species forming’ in nature. They are often surprised at the reaction they get from the better-informed creationists, namely that the creation model depends heavily on speciation.1
What is a species?
The size and shape of the beaks in these finch species are highly heritable. This follows one of the standard definitions of the word ‘species’: a group of organisms that breeds ‘true to type’. The problem with this definition is that there are many documented instances of hybridization among what were considered discrete species. Worse, most biologists follow the ‘biological species concept’ of [atheist and evolutionist] Ernst Myer (1904–2005). In his view, if two things can interbreed, they should be considered the same species. The problem with this definition is that scientists have documented chains of reproductive compatibility among, for example, all the living cats, but nobody wants to say that lions, panthers, lynxes, and house cats are one species. Creationists say they belong to the same created kind and that the various species are just subdivisions within that kind.
Our views on speciation and created kinds will affect our views on conservation, however, so we do need to be careful. It is generally not a good thing when species go extinct because the genetic variations they carry will be lost. This is one reason why captive breeding programs have been trying to save several of the near-extinct species of Galápagos tortoises. However, when two species merge, generally nothing is lost. Hybridization can also lead to a burst of change as traits that were once separate are brought together in new combinations. Thus, a merger of this sort, ‘despeciation’, is not necessarily bad. Indeed, the ancient (post-Flood) groups we call Neanderthals and Denisovans have disappeared, but many of their genes live on in modern humans due to ancient hybridization events (aka marriages).
In the case of Darwin’s finches, much change has been observed among them over the past 200 years. Some of the subspecies that were around in Darwin’s time are not with us today. For example, the larger form of the large ground finch (Geospiza magnirostris) from Charles Island has disappeared.2 Some of the other species have clearly changed (e.g., beak size has decreased) due to selective pressures, etc., as explained below.
Darwin’s finches are non-descript little birds that are endemic to Ecuador’s Galápagos Islands. They have been the subject of much speculation, including the urban myth that Darwin used them to support his early ideas about evolution. In fact, not only did he not properly identify the birds he collected while visiting the islands, but the thought that they might vary from place to place entirely escaped him, at least at first. The governor of the islands claimed that he could identify from which island any giant tortoise came, only based on the shape of its shell. Darwin dismissed this thought. Considering these birds, Darwin wrote in his Journal of Researches (1845), 20 years after the fact:
I did not for some time pay sufficient attention to this statement, and I had already partially mingled together the collections from two of the islands. I never dreamed that islands, about fifty or sixty miles apart, and most of them in sight of each other, formed of precisely the same rocks, placed under a quite similar climate, rising to a nearly equal height, would have been differently tenanted…
This explains why taxonomists back in England had to use the collections of others, notably that of the captain of the H.M.S. Beagle, Thomas Fitzroy, a Christian and future anti-Darwinist, to sort out Darwin’s collection. “Darwin’s finches?” Hardly.
New Study: Ground finch genome
Recently, however, a massive new study on these birds was published.3 It summarizes 40+ years of research among the four species of ground finch (genus Geospiza) that live on the tiny island of Daphne Major. Importantly, the researchers took blood samples from the birds they caught and tagged, starting in the 1980s, long before DNA technology was a thing. In this new paper, they report the full genome sequencing of nearly 4,000 birds, from all four species, that lived on this island over the past four decades. This is unprecedented work, and it illuminates multiple factors that contribute to the biblical creationist idea of speciation.
Peter and Rosemary Grant
Husband and wife team, Peter and Rosemary Grant, are the main drivers of this research. It was their vision to capture, measure, tag, and draw blood from every bird on that island over the course of multiple field seasons. They studied over 20,000 individual birds, one of which lived for 17 years, on this inhospitable little island from 1973 to 2012, right through several droughts (e.g., 1977, 1985, 2003–2005), during which up to 90% of the birds were wiped out, and excessively wet years (e.g., 1983). They made careful observations of which birds bred together and they tabulated long genealogical lists of the bird family tree. Their dedication to their craft is incomparable. I was introduced to their work in a book review by Dr Carl Wieland in 1995. I was a recent college graduate, a high school science teacher, and a brand-new subscriber to the Journal of Creation. His review of Jonathan Weiner’s The Beak of the Finch made me go to my local bookstore and order a copy for myself. I could not believe what I was reading. Instead of the slow-and-gradual, species change over millions of years mantra, I learned that species change very fast, and the Grants, though committed Darwinists, were documenting it. They published two popular-level books of their own, How and Why Species Multiply (2008)4 and 40 Years of Evolution: Darwin’s Finches on Daphne Major Island (2014). Both books are chock full of useful information for biblical creationists and are worth reading.
I had the pleasure of meeting the Grants once. I found them to be both amiable and interesting. In 2008, a CMI crew was flying to the Galápagos to start filming our Darwin: The Voyage that Shook the World documentary. I had been working for the ministry for less than two years and the ink on my PhD diploma was barely dry. I was sitting alone in my row, waiting for the airplane to take off from Guayaquil, Ecuador and take me to the islands. The last two people on the plane were an elderly couple dressed in weather-beaten clothing. I thought to myself that they looked like university professors on their way to a field station. The woman sat next to me. Her husband sat on the aisle. I worked up the courage to ask, “Are you Rosemary Grant?” She replied in the affirmative. I told her that I was a huge fan (which was, and still is, true, despite the fact that we have radically different views on origins), and we commenced a delightful and wide-ranging conversation that lasted for the duration of the two-hour flight.
In fact, Rosemary gave me a way to explain post-Flood biogeography. When we had taken off, she told me to look out the window and see if I could see any floating vegetation mats at the mouth of the Guayaquil River. I did see some small ones, but she said they can get up to a mile across and carry small animals, insects, and even leafy trees with them as they float out into the ocean. The creationist community has been talking about post-Flood ‘floating log mats’ for decades. I’m glad the evolutionists concur!
Table 1: Darwin’s finches (ground finches, genus Geospiza) and their locations among the Galápagos Island chain. The species that are endemic to Daphne Major are shaded. Not listed are the nine other finch species in the Galápagos: five tree finches (Camarhynchus spp.), two warbler finches (Certhidea spp.), the cocos finch (Pinaroloxias inornate), and the vegetarian finch (Platyspiza crassirostris).
Common name |
Species name |
Location within the island chain |
| Small ground finch | G. fuliginosa | Cosmopolitan |
| Medium ground finch | G. fortis | Cosmopolitan |
| Large ground finch | G. magnirostris | Near cosmopolitan west and north |
| Big bird lineage | (not yet named) | Daphne Major, central |
| Cactus finch | G. scandens | Near cosmopolitan |
| Española cactus finch | G. conirostris | Española Island and a few others in the SE |
| Genovesa ground finch | G. acutirostris | Genovesa Islands, northeast |
| Genovesa cactus finch | G. propinqua | Genovesa Islands, northeast |
| Sharp-beaked ground finch | G. difficilis | Central, west, and north islands |
| Vampire finch | G. septentrionalis | Wolf and Darwin Island in the far north |
The Grants’ research has borne much fruit.
- They have been able to see how species change in response to environmental perturbations (e.g., the periodic wet and dry years caused by the El Niño/La Niña cycle5).
- They showed that ‘change’ was fast.
- They noticed the arrival of the large ground finch (G. magnirostris) on the island in 1982 (three males and two females). This was followed by the migration of more individuals from a nearby larger island over the next several years (thus preventing a bottleneck event among the descendants of the founders). These birds outcompete smaller birds during droughts.
- They documented interspecies mating, notably when a female bird grew up in a nest near that of another species and ‘learned’ the song of the male in the other nest instead of that of her father. Since female birds tend to mate with males that sing like their fathers, when she flew off to find a mate, she picked a male from the ‘wrong’ species!
- They documented the appearance of a new species from the hybridization of two finch species, in a single breeding season, dubbed the ‘big bird lineage’. In 1981, a lone male Española cactus finch (G. conirostris), who was himself a hybrid of an Española cactus finch and a medium ground finch (Geospiza fortis), migrated to the island6 and mated with a female medium ground finch.7 The offspring mostly mated with each other (one female flew off to mate with a male cactus finch), and they have been doing so since their founding. They barely survived the 2003 drought, but they bounced back, despite being seriously inbred after this. These birds look different, act different, are genetically distinct, and have a different song than the other birds on the island. Even though there are not many of them, they would be called a different ‘species’ by all these measures.

New Study: Rapid speciation and hybridization
And now (with the publication of Enbody et al., 2023),3 the Grants and their team have shown how genes flow into, out of, and between species, affecting shape and behaviour over very short time periods (as opposed to Darwin’s concept that species change very slowly). This was facilitated by the small size of their study area and by the fact that there were but a few environmental and genetic factors at play. E.g., if you added feral cats to the equation (a common problem on small islands worldwide), most bird deaths would have been from predation and there would have been no way to see the effects of environmental change on the underlying genetics of the birds. The lack of predators on the island meant that competition for mates and food could more easily be traced back to the genes carried by the individual birds.
“The technical side”
This new study (figure 3) compliments the work of bird taxonomists (figure 4), who worked on this group for 200 years before anyone did a thorough DNA analysis. The branching patterns are a little off (e.g., G. magnirostris should branch off from G. fortis/G. fulginosa first), but the older classification schemes that were based solely on morphology identified the same species and species groups that we can see in the genetic data. Darwin, who so famously failed to properly label the specimens he collected, was told by the taxonomists back in London that the birds he collected were ‘good’ species.8 In other words, the collector thought he could easily separate them into discrete categories, and those categories have held up over time. These really are discrete species, by anyone’s definition.
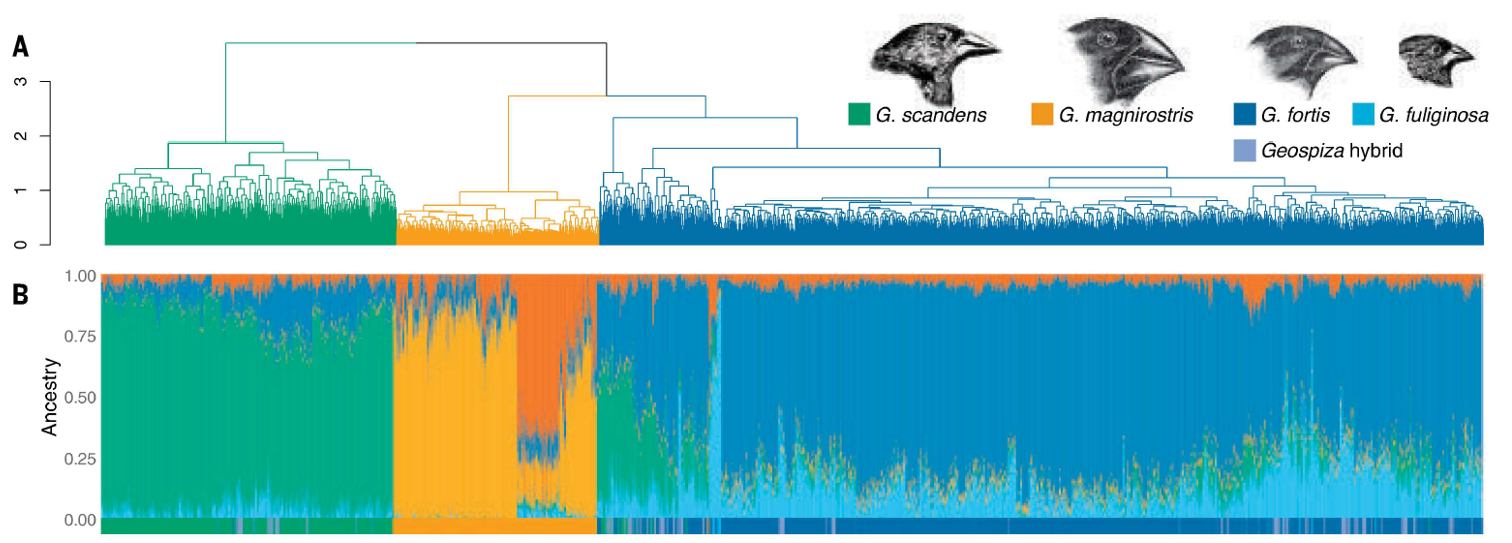

Enbody et al. were also able to show the slow introgression (‘gene flow’) of new genes from other species into the several Geospiza species on Daphne Major after the long drought years of 1980–1982. In fact, the birds were easier to distinguish at the beginning of the study (1970s) than at the end (2010s). In effect, strong natural selection during the drought led to specialization among the species. For example, the average beak size of the medium ground finch (G. fortis) demonstrably shrank during those years due to competition with the large ground finch (G. magnirostris). There is some variability in the size of the beaks in G. fortis, and the G. fortis birds that have smaller bills are better at getting to the smaller seeds. As the number of seeds on the island became more and more scarce, the large-beaked G. fortis birds could not keep up with the still larger-beaked and larger-bodied G. magnirostris. As a result, many G. fortis birds that had larger beaks perished, leaving behind G. fortis that had small beaks. Note that the birds did not ‘choose’ to have smaller beaks, nor did the beaks shrink. Instead, the large-beaked G. fortis birds died at higher rates, which changes the frequency of the genes that affect beak size and brought the average beak size down in the following generations. Later, a lessening of the selection pressure led to the species blending and the individuals becoming less specialized.
After much work, scientists have even been able to identify which genetic variants contribute to the size and shape of the beak and the overall body size of these birds (figure 5). There are a handful of variants that are strongly associated with the traits that vary among the species. Thus, just a few genes drive the speciation patterns, and we can see the effects of natural selection, mate choice, habitat choice, and several characteristic species behaviour patterns due to the presence of the various genetic variants.

Within the genomes of these birds, they found a handful of important genes that affect beak size, beak shape, and body size (figure 5). They also found one location with multiple genes that seems to have undergone no genetic rearrangement in a long time. They called this a ‘supergene’. Being that the natural world is wild and unconstrained, it is rare to find a non-laboratory system in which such things can be seen. Even more surprising is that there were but five to ten bird generations over the course of the study. In the Galapagos, we can see a strongly oscillating system with profound effects on the survival capabilities of various bird species. The reason we have not been able to document many other examples of change like this is that the world is generally chaotic and full of a multitude of stressors, most of which produce effects that are much less than life-or-death. Here, however, the selective signal was quite clear.
The species distinctions are also quite clear. Not only can the birds be separated according to features like body size and beak shape, in the family tree made from the genomes of the 4,000 birds sampled we can see clear separation between the various species. This is despite obvious ‘gene flow’ from one species to another. Here we have a group of species that can clearly hybridize, yet they mostly don’t. Behaviour, morphological specialization, song patterns, geography, and different sets of genes all act to maintain some level of differentiation among these members of the same biblical kind.
Natural Selection
When under stress, bacterial DNA mutates faster. This is a designed mechanism that allows them to explore new traits that might allow them to adapt. Generally, this creates a lot of mutant and poorly-functioning bacteria, but since there are so many of them in any given population, we often see, for example, antibiotic resistance traits arise, eventually. The type of mutation can also be influenced by environmental and genetic factors (e.g., C→T changes are much more common than A→G, but some genetic factors affect that ratio). Yet, there is no way for an organism to induce a specific letter change in a specific location unless there is a predesigned system for doing so. When we are talking about an entire genome of a bird species and many thousands of changes that have happened over time, we cannot appeal to some unknown mechanism that was designed to make specific changes. Instead, most of the new variation we see arising in all species is due to chance. Time + chemistry + imperfect DNA repair systems = mutation. This is fodder for natural selection.
Granted, God proscribed certain mutations when He designed the initial genomes at creation. He invented chemistry, and He determined which letters are more likely to mutate, so it was not like He would be surprised by DNA mutations. He coded specific letters into different areas of the genome so that some genes would not change and some would (e.g., hair, eye, and skin/coat colour changes are quite common in the animal world). God is also the author of created diversity. This refers to the genetic variation He programmed into the original created kinds, and the amount of created diversity could be significant.
When a genetic variant only appears in one animal, one species, or a small group of species within a baramin, we can be fairly certain that this is a new mutation, not created diversity. Thus, the genetic variants that affect beak size and shape among these birds are clearly mutations. Similar mutations, in the same genes, might exist in other members of this baramin, but the organisms did not choose to develop these traits. They inherited the traits and, now that we have sequenced the genomes of thousands of birds, each trait has a clear genetic basis (figure 5).
Yes, a bird has the ability to make choices about potential mates, where to live, and what to eat. But, that same bird has no control over the genes it carries or major environmental changes. There is also little evidence that epigenetics is at play in this instance. The connection between the underlying genes and the physical features of the birds is quite evident in the data. Thus, natural selection, acting on the underlying genes, is a real thing, and we can see it happening in these birds over time.
Designed to change and adapt
What does this tell us about creation? When God created the various ‘kinds’, He designed them to be able to change and adapt to a wide variety of circumstances. And it’s a good thing He did, for the modern world is quite different from the world before the Flood (at least, as far as we can tell from the fossil record). These birds are part of a group of organisms that were designed to be highly adaptable in size, behaviour, and shape (compared to other things, like the duckbilled platypus, the only living species in its genus and the only genus in its family). Thus, the fact that these birds change shape over time is a testament to the brilliance of the Creator. The evidence that Darwin and later Darwinists appealed to as support for their theory fits beautifully with biblical creation.
References and notes
- Wieland, C., Speciation conference brings good news for creationists, J. Creation 11(2):135–136, 1997. Return to text.
- Lack, D., Darwin’s Finches, Cambridge University Press, 1947, pp. 22–23. Return to text.
- Enbody, E.D., et al., Community-wide sequencing reveals 30 tears of Darwin’s finch evolution, Science 381:eadf6218, 2023. Return to text.
- Grant, P.R. and Grant, B.R., How and Why Species Multiply: The Radiation of Darwin’s Finches, Princeton University Press, 2008. Return to text.
- This is also referred to as ENSO, or the El Niño-Southern Oscillation. Return to text.
- He had flown over 100 km (60 miles) from Española Island and had to cross or fly around the large island of Santa Cruz to get to Daphne Major. Return to text.
- Lamichhaney, S. et al., Rapid hybrid speciation in Darwin’s finches, Science 359:224–228, 2018. Return to text.
- This is a word we see used by taxonomists from time to time. A ‘good’ species just means that it can be easily separated from other, similar species. Return to text.



Readers’ comments
Comments are automatically closed 14 days after publication.